CDMOs that provide finish to finish progress and manufacturing providers have the ability to integrate lyophilization routines and supply a seamless changeover from drug improvement to commercial-scale creation, making sure consistency and top quality at just about every stage.
Although plant-centered foods are the most popular freeze-dried products, a variety of foods could be preserved using this method.
X Get item sheets By now a customer of Demaco Holland B.V.? You already have entry to the connected files but feel free to submit a completely new ask for.
Modern innovations are reshaping the lyophilization landscape. Examples consist of automatic loading and unloading devices that lessen the risk of contamination and human mistake; Sophisticated freeze-drying sensors and process analytical technological innovation (PAT) tools that make it possible for for better control of the lyophilization process; managed nucleation procedures that standardize the freezing stage in the process; improvements in Vitality efficiency and therefore Value; devices and process Handle improvements that permit successful and predictable scalability from lab to industrial scale; integration with application and info analytics to aid much better process being familiar with and optimization; as well as adoption of high-quality by layout (QbD) ideas that help a lot more robust and productive lyophilization processes.
Whilst lyophilization remains the best choice for your Risk-free storage and usage of biologics and tiny molecules, there are a few challenges and limitations due to the advanced process outlined over. In line with Mirasol at Biopharm Intercontinental, this elaborate process can become much more challenging, depending on what Organic molecules are inside the solution alone.
Our professional facility functions sterile lyophilization and is also integrated into our present advancement and scientific demo producing site, featuring buyers a seamless flow from enhancement by way of producing.
Lyophilization, often called freeze drying, is usually a process used to protect thermolabile elements like pharmaceuticals and food stuff by eradicating h2o through the materials after they are frozen. The process entails freezing the material, lessening strain to allow the frozen h2o to sublimate directly from the stable period to fuel stage, and then working with lower temperatures and tension to remove remaining drinking water.
Know when your samples reach dryness. Arrange your Conclude-Zone™ Conclusion Level Detection Technique to provide you with a warning when the first drying section is comprehensive for as much as 5 samples in 600ml or larger sized flasks. This movie shows how to set up and work the program.
cryopreservation of pharmaceuticals freeze drying pharmaceuticals QbD scale up pharmaceutical processes biopharmaceuticals biologics drying technologies pharmaceutical sciences antibody drug conjugates CART (chimeric antigen receptor modified T-Cell) BITES (Bispecific T Cell ) Search inside of this reserve
Stress is lowered after which you can warmth is placed on change the frozen water into vapor. Manufacturers must watch out to use barely enough heat to forestall melting and damaging the fabric [*].
Being an inaugural member from the Process Advancement team, Matt now manages highly expert scientists in the identical team, leveraging his process know-how and complex prowess to inform experts and shoppers alike, from compact scale preclinical exams to late-phase characterization and aseptic fill-finish. Matt been click here given his B.S. in Chemical Engineering through the College of Massachusetts.
While in the secondary or ultimate drying stage, the residual moisture articles is decreased just as much as feasible to ensure that the products is within a completely storable condition. The h2o sure by adsorption at The interior area of your product must be removed. To obtain this, it is often essential to conquer h2o’s capillary forces.
If your vacuum is broken click here with untreated sterile air, the products could absorb oxygen and drinking water vapor. Following pre-aeration is entire, the stoppers are sealed in to the vials under a slight vacuum. Following the stoppers are completely shut, the vacuum is entirely damaged with sterile air.
The containers may be sealed beneath vacuum or a protective gas atmosphere. The selection of process depends upon solution.
 Danny Tamberelli Then & Now!
Danny Tamberelli Then & Now! Josh Saviano Then & Now!
Josh Saviano Then & Now! Michael Oliver Then & Now!
Michael Oliver Then & Now!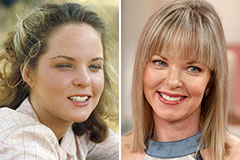 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Lacey Chabert Then & Now!
Lacey Chabert Then & Now!